Why are we concerned with Quality Risk Management (QRM) when planning, developing, and executing commissioning, qualification, and validation work?
The simple answer is time and money. Pharmaceutical companies are under pressure to bring new therapies to market quicker and to do so at less cost, while still meeting the mandates of cGMPs which ensure the Safety, Integrity, Strength, Purity and Quality of the manufactured medicine. Incorporating a Quality Risk Management approach when designing a validation strategy helps to ensure that the testing is focused on the right things (factors that provide assurance that SISPQ has been met) and at the appropriate level based on sound science and product/process understanding.
Strategies using risk management can be found in several approaches to commissioning, qualification, and validation work:
- ISPE Baseline Guide 5 (2007) embraces the use of system and component classifications to help frame the qualification in terms of system impact to product quality and patient safety (direct, indirect, no impact) and component classification (critical/ non-critical). Systems and components that have a direct impact to product quality should be subject to the greatest rigor of testing and level of review. Items that are indirect or no impact still need to be tested for ensure proper operation and suitability of use – but this testing should be commensurate with the level of risk to product quality and patient safety.
- ASTM E2500 (2013) incorporates a risk-based approach as identified in ICH Q9. This includes the evaluation of the risk to quality based on scientific knowledge and ultimately linked to patient safety (sec 6.2.2.1). The level of effort, formality, and documentation of the verification process should be commensurate with the level of risk (sec 6.2.2.2). Additionally, the principles of risk management should be applied to specification, design, and verification of the manufacturing systems.
Risk management approaches are described in various ICH Documents (ICH Q7, Q8, Q9, and Q10) and FDA documents ( Jan 2011 Guidance for Industry on Process validation, and Pharmaceutical cGMPs for the 21st Century – A Risk-Based Approach).
ICH-Q8 Pharmaceutical Development (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use [ICH], 2009) describes a typical pharma development approach (Figure 1). Risk tools as illustrated in ICH Q9 (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use [ICH], 2005) may be used to flow chart the manufacturing process to identify the raw material, product, and people flows and then through use of an Ishikawa diagram to identify how these inputs may influence a critical quality attribute. An example of the use of the Ishikawa diagram is shown in Figure 2. Once the risks are identified, risk ranking techniques (e.g. Failure Mode Effects Analysis (FMEA)) may be used to help identify the criticality of the attribute or process parameter and thus aid in the development of the validation program and control strategy for the product manufacturing lifecycle (See Control Strategy, Enhanced Training Elements.)
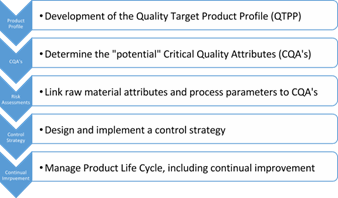
Figure 1. Typical Pharmaceutical Development plan integrating ICH Q8, 9 and 10. Adapted from Training Programme for Q8/Q9/Q10 work products developed by the ICH working group (Implementation of ICH Q8, Q9, Q10, by ICH (2010), retrieved from www.ich.org.
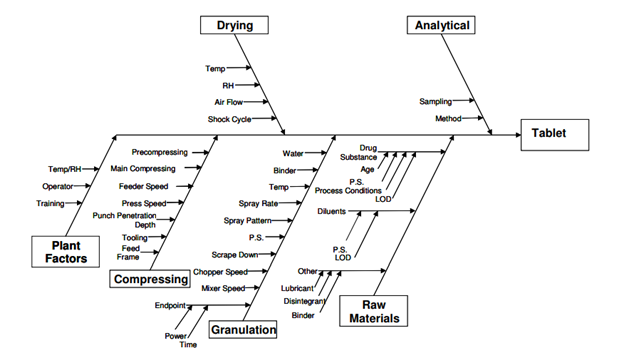
Figure 2. Example Ishikawa diagram. Retrieved from Appendix 2, Illustrative Examples ICH Q8 (2009)Pharmaceutical Development, developed by the ICH working group retrieved from www.ich.org
However, it is important to understand that risk management is not limited to the use of quality tools, but is a process whereby a multifunctional group representing various stakeholders participate in the process of risk assessment (identification, analysis, and evaluation), followed by control (risk reduction and acceptance) and that the output of the risk management process is effectively communicated (as necessary and appropriate) through the organization. Risk communication ensures that key organizational members who may not be directly involved in the risk assessment process are aware of the outputs of the risk management process. The Risk Management process is illustrated in Figure 3.
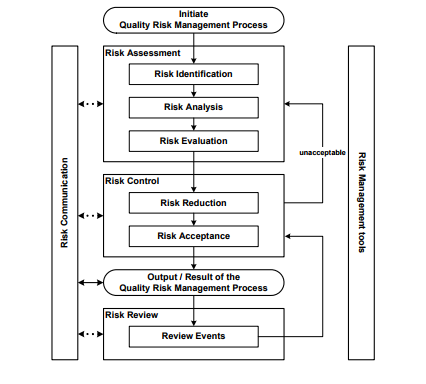
Figure 3. Overview of a Typical Risk Management Process retrieved from ICH Q9 Quality Risk Management (2005)
Annex II of ICH Q9 (2005), identifies 8-potential applications of quality risk management and how quality risk management may be used as part of an integrated quality system, or throughout the product quality lifecycle.
One specific example would be performing quality risk management for facility design. As identified in Annex II of ICH Q9 (2005), this may include HVAC zone classification for protection of raw materials, personnel and product, qualification of equipment, facility, and utilities, cleaning and environmental control, calibration and preventive maintenance, and computer systems.
In summary, quality risk management incorporates various quality tools (Annex 1, ICHQ9), that are used as part of a multifunctional interrogative process that performs risk identification, assessment, and evaluation of impact to product/patient quality. The QRM process accepts risks that are at or below a user implemented threshold, mitigates risks that are above this threshold to a lower acceptable level, and if necessary accepts the residual risks. The QRM team participates in periodic or event based risk reviews and communicates the results of the risk management process to the organizations management.
Have a question on Quality Risk Management? Please use our “Contact Us” form to get in touch with one of Performance Validation’s experts.
References:
ASTM International. (2013). E2500-13: Standard guide for specification, design, and verification of pharmaceutical and biopharmaceutical manufacturing systems and equipment. West Conshohocken, PA: ASTM International.
International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use [ICH]. (2005). Quality Risk Management: Q9. Retrieved from https://database.ich.org/sites/default/files/Q9_Guideline.pdf
International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use [ICH]. (2009). Pharmaceutical Development: Q8. Retrieved from https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf
International Society of Pharmaceutical Engineering [ISPE}. (2007). Baseline Guide: Volume 5 Commissioning and Qualification (Vol. 5). Tampa, FL: ISPE.
