Medical devices must meet stringent regulatory requirements to ensure patient safety. However, the demand for quicker medical device production makes this a challenge, as compressed timelines allow for more production variance, resulting in subpar outcomes. CQV (Commissioning, Qualification, and Validation) ensures medical devices follow design specifications and operate in accordance with predetermined standards, regulatory requirements, and quality standards throughout their lifecycle. Medical device validation utilizes documented evidence to verify consistent adherence to pre-established quality, safety, and performance criteria, ensuring compliance with regulatory requirements. Through validation, you can:
- Minimize the risk of defects or malfunctions that could compromise patient safety.
- Identify and rectify any issues during the development and manufacturing stages.
- Comply with regulatory bodies, such as the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
- Obtain documented evidence that your manufacturing processes are consistent and reliable.
Medical Device Validation in Three Steps
The medical device validation process is critical for meeting regulatory standards. With this three-step approach, you can reduce costs and shorten project timelines without sacrificing quality or compliance.
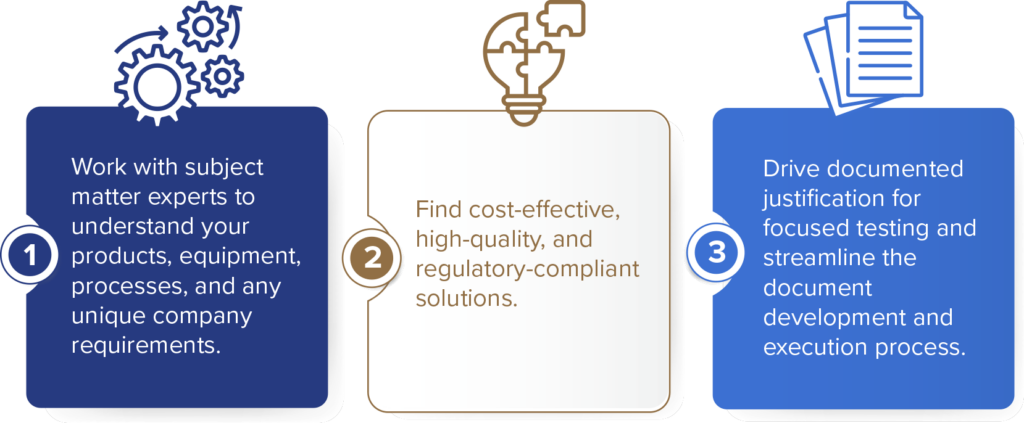
We use a best-practice approach incorporating ISO concepts while helping develop procedures that address other regulatory needs, including:
- Management controls
- Design controls
- Corrective and preventive actions
- Production and process controls
Better Outcomes Start with Better Planning
PV offers comprehensive services to ensure the quality, safety, and regulatory compliance of medical devices. When you require equipment qualification, process validation, test method validation, or connected device services, trust us to get the job done compliantly.
- Macro validation planning and reports
- Risk analysis development
- Project management (including expense, project, and milestone management)
- Process validation expertise
- Class I, II, and III medical device manufacturing
- New production lines
- Existing production line updates
- Quality system development
- Validation program governing documents
- Corrective and preventative action procedures
- Production and process control procedures
- FDA remediation
- Form 483 response and completion
Medical Device Process Validation Services that Meet Compliance
PV specializes in medical device process validation, offering expert support in developing and executing high-quality operational and performance qualification (OQ/PQ) protocols. We work to understand, document, and test your manual, automated, and special processes for compliance.
- Laser marking
- Laser, ultrasonic, and resistance welding
- Adhesive curing
- Soldering
- Packaging
- Injection molding
Reliable Test Method Validation Services
PV recognizes the need to ensure a test method is suitable for its intended use. We work with quality systems to develop a sampling plan and acceptance criteria before statistically analyzing results.
- Tensile testing
- Compression testing
- Automated measurement systems
- Visual inspection
Quality System Regulation (QSR) Support
When you need quality system regulation (QSR) support for medical devices, trust our team to deliver satisfactory results. Get help developing, implementing, and maintaining quality management systems that follow the regulatory requirements outlined in the QSR. Auditing processes or elements we’ve helped refine include:
- Validation program governing documents (e.g., production equipment qualification, facility qualification, process validation, cleaning validation, computer system validation, analytical equipment qualification and methods qualification)
- Supplier assessments of new or current vendors
- Qualification and validation packages
- Design history file
- Change control
- Batch record reviews
Improving Patient Safety Through Connected Device Services
Connected medical devices are already entering everyday life, bringing the potential to deliver better patient care and improve operational efficiency. Medical device connectivity can help provide healthcare professionals with complete, accurate and current patient assessments on demand. At PV, we understand the importance of patient safety and offer Computer Software Validation and Software as a Medical Device (SaMD) solutions. Our team also has experience in validating artificial intelligence (AI).
Your Dedicated Partner for CQV Pharma Services
PV supports Fortune 500 companies, foreign subsidiaries and smaller medical device companies with their medical device validation needs. Let us help you define equipment requirements, installation qualifications, and final assembly processes to ensure compliance with industry regulations and standards.
Frequently Asked Questions
Medical device CQV (Commissioning, Qualification, and Validation) ensures that medical devices meet regulatory requirements and operate according to predetermined standards, enhancing patient safety and product quality.
PV develops tailored validation protocols that align with the regulatory requirements set forth by agencies like the FDA and EMA, ensuring that all documentation and processes are compliant.
Packaging validation guarantees that devices are securely sealed, protected from contamination, and compliant with industry standards throughout their lifecycle.
Software validation ensures that all software components function correctly and safely, helping to prevent compliance issues and enhance overall patient safety.
Design validation involves assessing specifications, conducting user testing, and analyzing performance to ensure the product meets user needs and regulatory standards before market release.
By thoroughly documenting and testing manufacturing processes, process validation helps identify and resolve issues early, minimizing the risk of defects and improving overall product reliability.
PV offers validation services for various Test Methods. PV has performed Test Method validations for attribute and variable GR&R studies.
PV provides validation for connected devices, including computer system validation, ensuring they are safe and effective for patient care.
PV supports a range of clients, including Fortune 500 companies, foreign subsidiaries, and smaller medical device companies, helping them meet their validation needs effectively.
